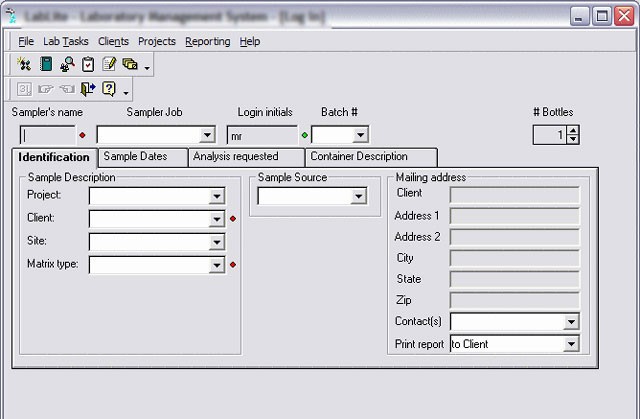
LIMS – Laboratory Information Management System
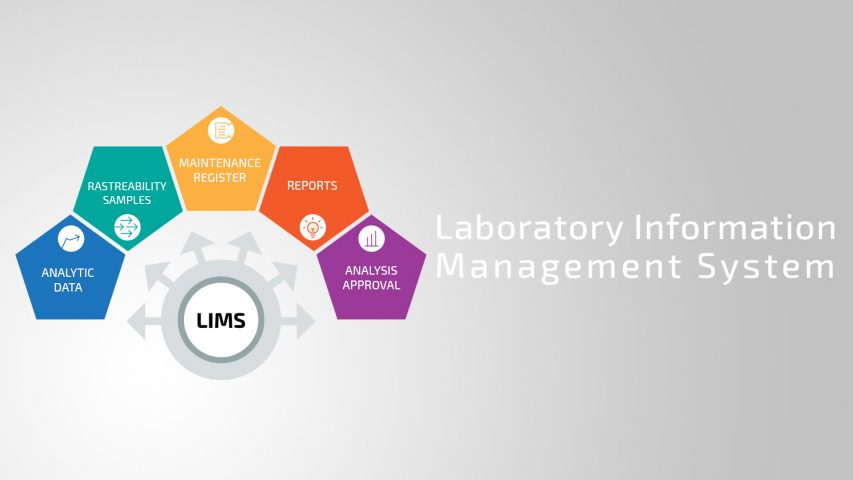
LIMS - Laboratory Information Management Systems are quality control laboratory systems responsible for collecting and managing the flow of analytical data, approval of analyzes, traceability of samples and reagents, maintenance records, etc..
They may have interfaces with chromatographic data systems, analytical instruments, reports, other systems, etc.
Due to its characteristics, LIMS has been increasingly used, making it possible to increase productivity and reduce operating costs.
Before undertaking the system selection, however, the company must have clearly defined project requirements such as database, norms compliance, laboratory needs, project start and finish forecast, and other pertinent information.

The validation of a LIMS - Laboratory Information Management Systems
The LIMS system when well implemented brings innumerable advantages for the company. Comparatively, we can affirm that LIMS is like an ERP (Enterprise Resource Planning) management system for Quality Control. It manages all laboratory flow from sample input, results, issue of analysis bulletins, reporting.
One of the characteristics that makes the LIMS system complex to implement and validate would be the interfaces between systems. Overall, it has the potential to integrate with lab equipment and ERP. The prospective validation, that is, performed during the routine implementation of the system, can greatly help to reduce the difficulties of the Project phase.
The mapping feeds the Risk Analysis scenarios. In a multidisciplinary team, the risks should be analyzed, if possible, involving LIMS’ suppliers and the main equipment that will be integrated, such as HPLCs (High Performance Liquid Chromatography).
The result of the Risk Analysis would nourish the URS (user requirements). When it’s the planning phase is finished, then follows the life cycle, composed of Functional Specification, Hardware Design, test protocols, Traceability Matrix and Validation Report.
See here, the Document Life Cycle